 Admin
Admin Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals
| Author List |
|---|
| Andrew Hill |
| Loai Tahat |
| Mohammed K Mohammed |
| Rabab F Tayyem |
| Giten Khwairakpam |
| Sanjay Nath |
| James Freeman |
| Ismahane Benbitour |
| Sherine Helmy |
Abstract
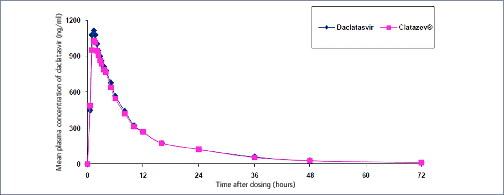
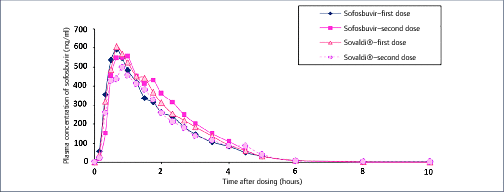
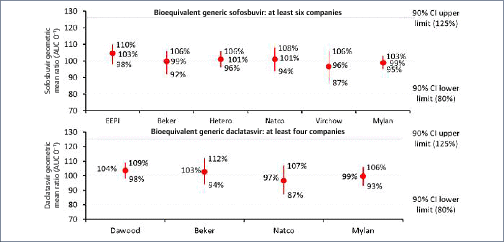
Mass production of low-cost, generic direct-acting antivirals (DAAs) will be required to achieve targets of eliminating hepatitis C (HCV) by 2030. The pharmaceutical companies Gilead and Bristol-Myers Squibb have granted voluntary licences (VLs) to generic companies to mass produce the DAAs sofosbuvir and daclatasvir at low cost. However, generic manufacturers need to demonstrate bioequivalent pharmacokinetics for their DAAs, compared to the originator versions, to fulfil World Health Organization standards for prequalification. The aim of this study was to determine whether generic forms of sofosbuvir and daclatasvir had bioequivalent pharmacokinetics to the originator versions.
Published
Article Category
Hepatitis C (HCV)
Article Type
Short communication
Posted Date
29-03-2018